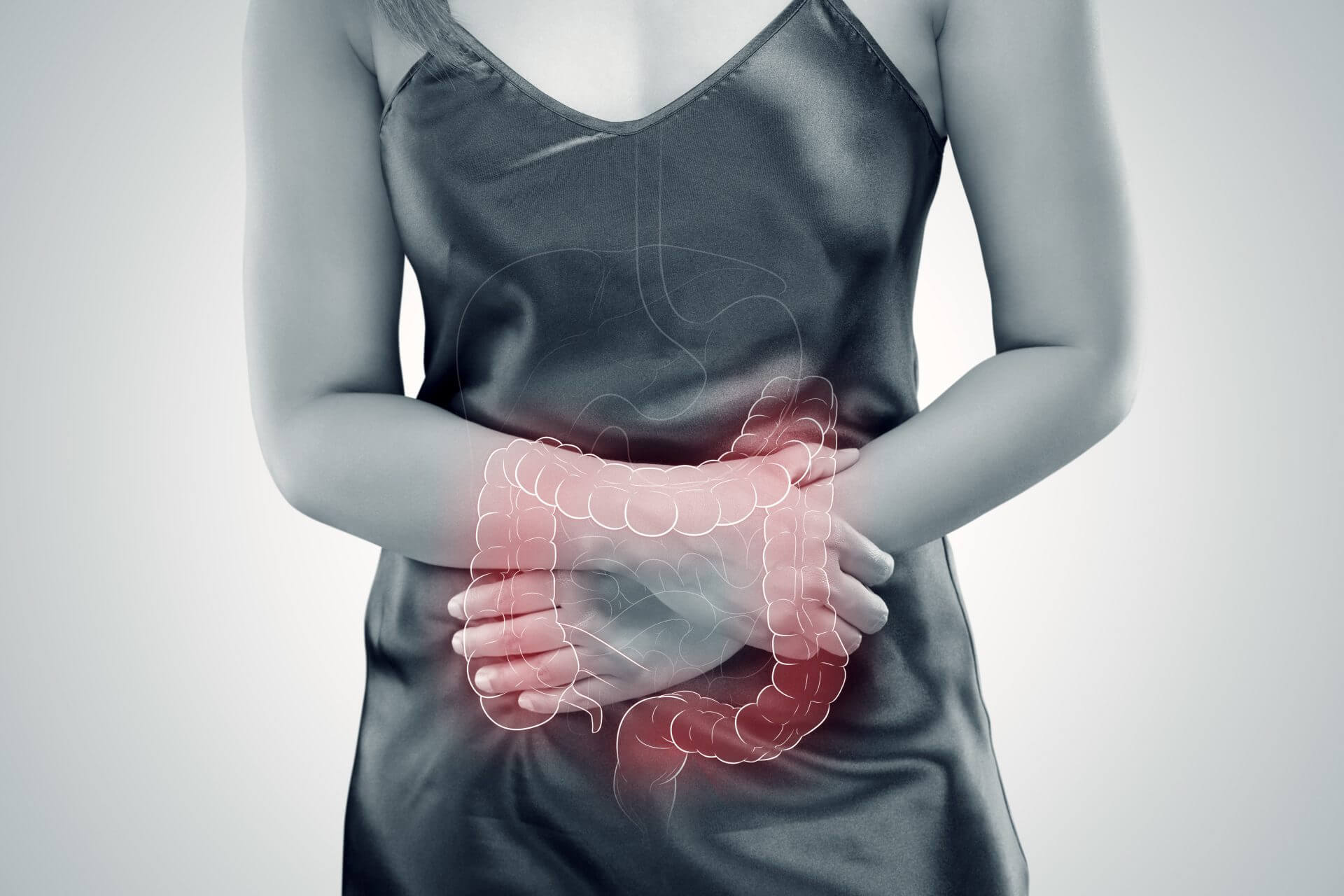
Lipid Therapeutics today announced that it has entered into a licensing agreement with Nestl’ Health Science for exclusive rights to Lipid Therapeutics’ LT-02 compound (phosphatidylcholine), a novel barrier function therapy for patients with mild-to-moderate ulcerative colitis (UC), worldwide excluding Europe and Australia. The terms of the license include an upfront fee, milestones payments and royalties on net sales.
Under the agreement Nestl’ Health Science is responsible for the continued development and commercialisation of LT-02 in its territories, with Phase III clinical trials in the US planned to start in 2016. These trials will primarily assess LT-02 as an add-on therapy in patients with UC not adequately responding to standard doses of 5-aminosalicylic acid, the current standard treatment for the condition.
In October 2014, Lipid Therapeutics’ European co-development partner, Dr. Falk Pharma GmbH, a pharmaceutical company specialized in gastroenterology, announced the start of a pivotal Phase III trial with the same compound in Europe. The European and US Phase III trials form part of a global Phase III development programme that aims to provide clinical evidence to support regulatory approval of LT-02.